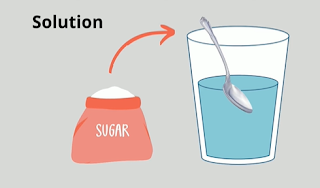
A solution is a mixture of two or more substances.
Properties of a Solution
The particles in a solution cannot be seen.
Light is not visible when it passes through a solution.
A solution cannot be separated with a filter.
A solution is a homogenous mixture.
An example would be sugar and water. The sugar is the solute and the water is the solvent. The solute is the item being dissolved and the solvent is what the item is being dissolved into.
A suspension is a mixture of a fluid with undissolved particles in it.
Properties of a Suspension
The solid particles may be visible and will settle over time.
The particles of a suspension can be filtered out.
Light is visible when it passes through a suspension.
A suspension is considered a heterogenous mixture.
The solid particles are called the internal phase and the fluid is called the external phase.
An example would be muddy water and sand in water. The sand does not dissolve into the water but is mixed into the water and will settle over time.
A colloid is a mixture consisting of very small insoluble particles dispersed into a fluid.
Properties of a Colloid
Light is visible when it passes through a colloid.
You cannot use a filter to filter out what is dispersed and the particles do not settle over time.
A colloid is a homogenous mixture.
Examples of a colloid include, glue, milk, and fog.
A colloid has a dispersed phase (the suspended particles) and a continuous phase (the medium of suspension).The continuous phase can be a solid,liquid,or a gas.




0 comments:
Post a Comment