Saturated, Unsaturated and Supersaturated Solutions
Let's take a solvent like water and mix in salt at a given temperature.
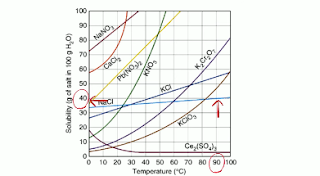
Water can only dissolve so much salt you can look these up or use a solubility curve.
When water is at 90 degrees Celsius it can dissolve 40 grams of salt in 100 grams of water. The water is the solvent and the salt is the solute.
When you have an unsaturated solution less solute then the solution is capable of dissolving is mixed in.
Solutes in an unsaturated solution dissolve completely.
Using salt as an example, if we mix in 20 grams of salt at 90 degrees it will be unsaturated. All of the salt will be dissolved.
With a saturated solution there is so much solute present that if you were to add any more it will not dissolve and excess salt will fall to the bottom of the container.
If you mix in 40 grams of salt at 90 degrees into 100 grams of water.
A supersaturated solution contains more than the maximum amount of dissolved solute then the solvent is able to dissolve at a given temperature.
If you mix in 50 grams of salt the extra salt will sink to the bottom of the container.


0 comments:
Post a Comment