Let’s take a look at the difference between reactivity and combustion.
Reactivity is the chemical reactions of a single substance or two or more substances that interact with each other. Highly reactive substances may react explosively, while other reactions may include fizzing, or a change in color.
Reactivity depends on the arrangement of the valence electrons.
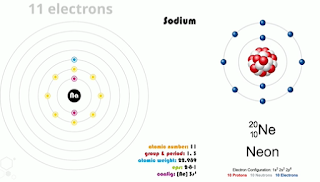
Let’s look at sodium and neon for understanding of this concept.
Sodium has 11 electrons and neons has 10.
They both have 2 electrons in the inner shell and 8 in the second shell but sodium has one electron in its outer shell. Elements desire to have their shells filled up so neon is happy with 8 electrons in it’s outer shell. Sodium has only one electron in it’s outer shell so it is very unhappy. Sodium can easily lose one electron in its outer shell in a chemical reaction so it is highly reactive.
Combustion is a chemical process in which a substance reacts rapidly with oxygen and gives off heat. The original substance is called the fuel, and the source of oxygen is called the oxidizer.
The fuel combines with oxygen and releases energy in the form of heat, light, and sound.
Two Combustion Reactions
Coal + oxygen yields carbon dioxide + energy
Hydrogen + oxygen yields water + energy
This can be an explosive reaction if you add heat.






0 comments:
Post a Comment