How to Balance Chemical Equations
A chemical equation is balanced when you have the same number of atoms for the reactants and the products.
You can balance chemical equations by following these simple steps.
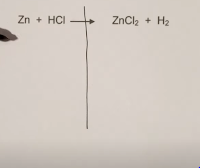
Step 1. Draw a vertical line from the arrow.
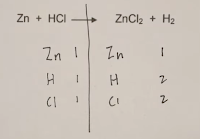
Step 2. Count the number of atoms for the reactants and the products.
When you have a coefficient in front of one or more elements you multiply the coefficient for all of the elements. A subscript only applies to the element it is written at the end of. The coefficient will also be multiplied by the subscript.
Step 3. Start with the metals and use coefficients so that the number atoms are equal for reactants and products. On the periodic table the metals are found on the left and the transition metals are in the middle and nonmetals are on the right.
Remember you cannot change subscripts only coefficients.
Step 4. Get the number of nonmetal atoms balanced for the reactants and products. Again, you can only change the coefficients and not the subscripts.
Step 5. Balance the remaining elements like hydrogen by changing coefficients.
If you have two products and reactants unequal then try this. Find the smallest number they both divide evenly into and then divide the subscripts into this number.
For example, you have 3 Chlorines on the reactant side and 4 Chlorines on the product side.Then 12 is the smallest number they both divide into. Once you have this number you can divide the subscripts into this number in order to know what the coefficient needs to be.
You would have a coefficient of 4 on the ractant side, and a coefficient of 3 on the product side.
Additional Resources





0 comments:
Post a Comment