Hydrocarbons are often considered the simplest organic compounds and contain only carbon and hydrogen.
Let’s take a look at four different types of hydrocarbons
The alkanes, the alkenes, alkynes, and cyclic.
Alkanes contain only single bonds.
The general formula for alkanes is CnH2n + 2
Let’s look at two examples.
Methane is CH4 following the formula you have 1 carbon and for hydrogen, you take 1 x 2 + 2 = 4
Another example of a Alkane is Butane C4 H10
Hydrogen = 4 x 2 = 8 + 2 = 10
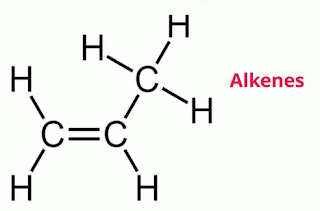
Alkenes contain at least one double bond.
The general formula is Cn H2n
Let’s look at two examples.
Propene is C3H6
Hydrogen = 3 x2 =6
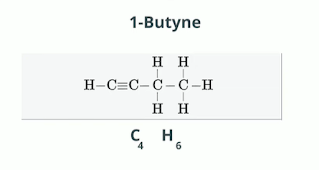
Alkynes contain one or more triple bonds.
The general formula is Cn H 2n-2.
Two examples are
Propyne C3H4
Hydrogen = 3 x 2 = 6 -2 = 4
Butyne C4H6 4 x 2 =8 -2 = 6
Cyclic hydrocarbons have a carbon ring.
The general formula is CnH2n
Hydrogen = C4H8
Hydrogen = 4 x 2 =8
You may also enjoy ...




0 comments:
Post a Comment